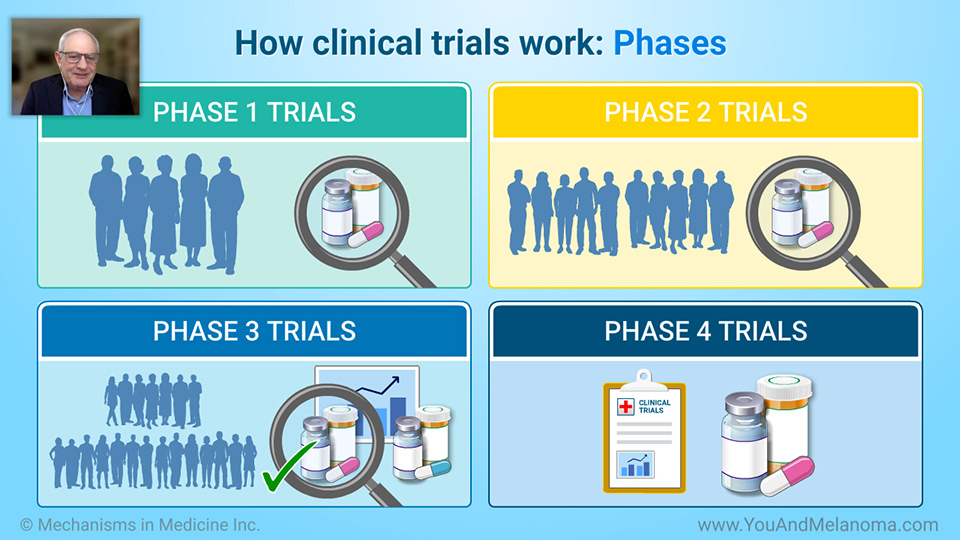
Melanoma experts Dr. Sapna Patel and Dr. Michael B. Atkins discuss what happens after a
melanoma clinical trial is completed. Dr. Patel also discusses considerations for when a participant can stay on clinical trial treatment and what happens if the FDA approves a clinical trial treatment for melanoma. In addition, Dr. Akins discusses successful immunotherapy clinical trials for melanoma and
data safety monitoring of melanoma clinical trials.
-
Share with family and friends:
Click here to take our SURVEY
Your feedback is important to us! We will use your feedback to develop future areas of content about melanoma which will help other patients, caregivers, and families.