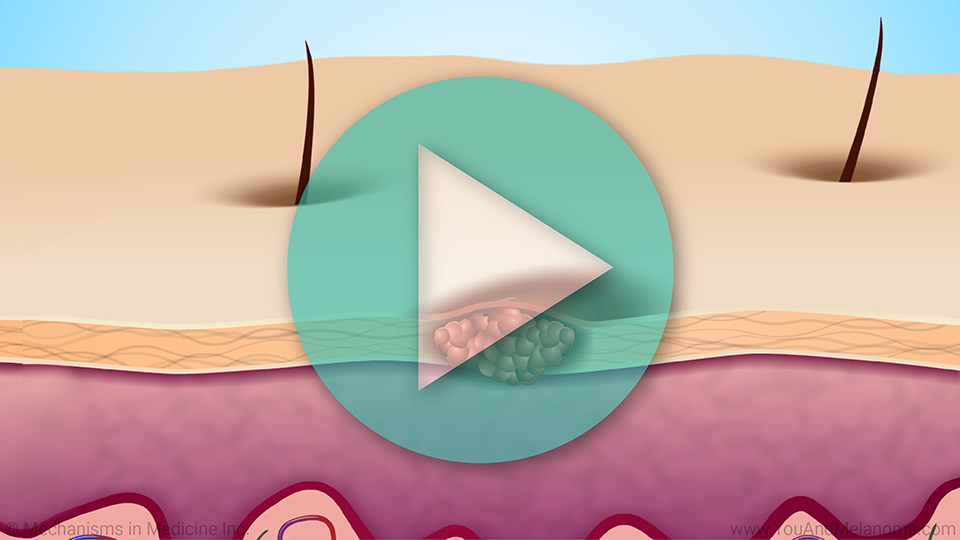
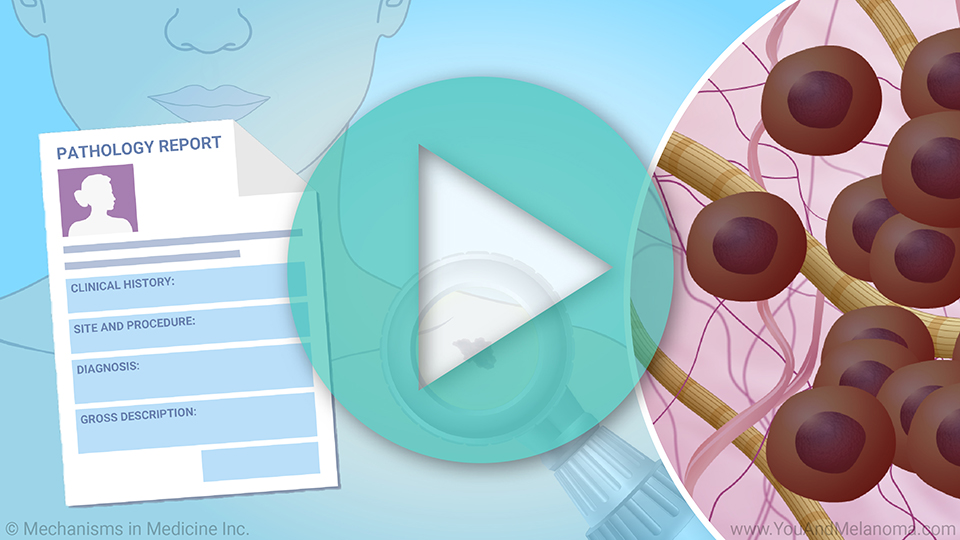
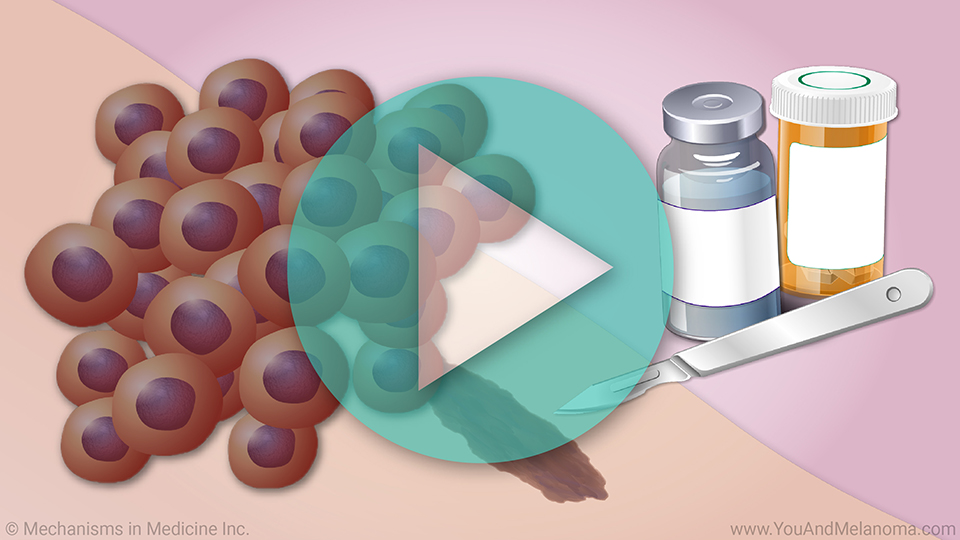
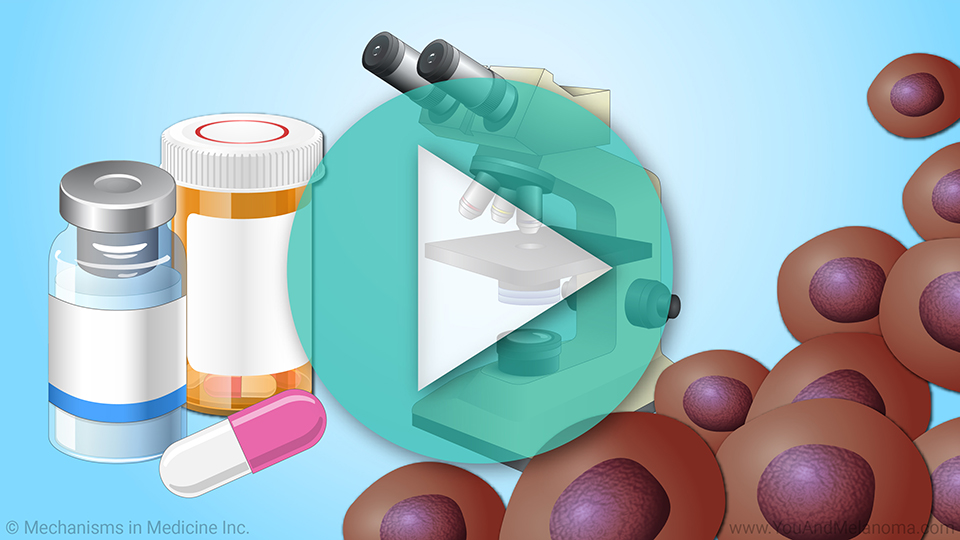
This animation provides an overview of
Tumor Infiltrating Lymphocyte (
TIL)
Cell Therapy for
melanoma. TIL Cell Therapy is a
new type of immunotherapy that utilizes TIL to attack cancer cells. TIL Cell Therapy is now an FDA-approved treatment for non-uveal melanoma. In addition, researchers are conducting many clinical trials of potential new TIL treatments. TIL Cell Therapy is approved for people whose tumors have spread or cannot be surgically removed and who may have already had certain other melanoma treatments. Watch to learn how TIL Cell Therapy is
prepared, how the
infusion works, its
effectiveness, possible
side effects, and its current use in
melanoma. You may want to ask your doctor if TIL Cell Therapy could be an option for you.
-
Share with family and friends:
Click here to take our SURVEY
Your feedback is important to us! We will use your feedback to develop future areas of content about melanoma which will help other patients, caregivers, and families.